About Nikkei article usage service
If you would like to share articles with your company, reprint/duplicate in conference materials, or order printing, please see the link.
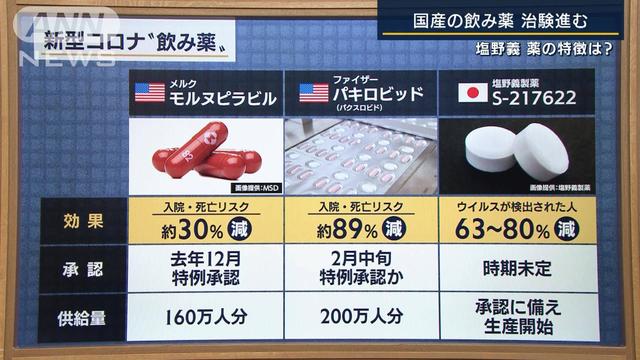
Click here for detailsPfizer, a major US pharmaceutical company, announced on the 17th that it has agreed with the Japanese government to supply 2 million doses of the new coronavirus drug under development. Clinical trials (clinical trials) are being conducted in Japan as a therapeutic drug for mild to moderately ill patients. If Pfizer's products are supplied in addition to Merck's oral medicines, which have already been decided to be supplied to Japan, the options for treatment will expand.
"Paxlobide" under development by Pfizer is a method of combining "PF-07321332 (development number)" developed for Corona and the antiviral drug "ritonavir". It is a mechanism that inhibits the replication of viruses inside cells.
Pfizer will begin human trials this spring. According to the clinical trial data released on the 14th, the risk of hospitalization and death in infected people who were at risk of becoming seriously ill who received the drug within 3 days after the onset of symptoms was reduced by 89%. According to Pfizer, initial laboratory analysis confirmed that even the new variant, the omicron type, was effective in preventing the virus from multiplying.
Pfizer is discussing with the Ministry of Health, Labor and Welfare and others to apply for manufacturing and marketing approval necessary for practical use. If approved, the drug will be provided to the Japanese government for treatment of 2 million people. In the United States, an emergency use authorization application was filed with the Food and Drug Administration (FDA) on November 16.
In the oral medicine developed for the new corona, US Merck's "Mornupyravir" has already been put into practical use in the UK and other countries. Prior to commercialization in Japan, Merck agreed with the Japanese government to supply approximately 1.6 million servings for approximately $1.2 billion (approximately 130 billion yen). Pfizer's paxlobide works differently than molnupiravir, so if the two companies' products are commercialized, they could be used by a wider range of patients.
Open in the appAll articles are unlimited reading Paid members are free for the first month
About Nikkei article usage service
If you would like to share articles with your company, reprint/duplicate in conference materials, or order printing, please see the link.
Click here for detailsNew Corona
You can read all about the new coronavirus here. Vaccines/therapeutic drugs, leave of absence/compensation, business, domestic, overseas, infection status
Related Topics
Following topics makes it easier to check what's new and read more.
Also recommended (automatic search)
Related keywords
